- When patients with dysglycemia had severe hypoglycemia while on treatment with basal insulin glargine, the episodes did not trigger as many adverse cardiovascular outcomes as did severe hypoglycemia during standard care with oral glucose-lowering drugs but no insulin.
- "Hypoglycemia caused by insulin glargine–mediated glucose lowering is unlikely to cause cardiovascular outcomes," Dr. Linda G. Mellbin said at the European Society of Cardiology Congress 2013.
- "What was reassuring was that while insulin glargine caused more hypoglycemic events" compared with standard treatment in these patients, "the absolute number of fatal events was still lower with insulin, showing insulin is a very safe treatment," said Dr. Lars Rydén, professor of cardiology at the Karolinska Institute and a coinvestigator with Dr. Mellbin.
Counterpoints:
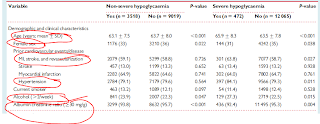
This subanalysis does very little to answer the following paradox. Despite having more severe hypoglycemias(76% vs 24%)-consistent and robust relationship to all primary and secondary endpoints-in the glargine treated arm, there is no excess mortality in the treatment arm. They are offering the commentary that glargine is a safe insulin regimen and that people should not be reluctant to start insulin early.
- This study highlights the clear relationship between severe hypoglycemias with death and cardiovascular mortality. There are more severe hypoglycemias with lantus than standard oral therapy. [This highlights the clear added risk when you introduce lantus for therapy.] There is a 3 fold risk of inducing symptoms so severe that you need assistance.
- People had been supporting glargine through claiming that it is a smooth, peakless insulin. In this study, it shows that glargine is not better than stardard of care sulfonureas. It does not provide any cardiovascular advantage nor is it safer than sulfonureas.
- We know from the UKPDS that early aggressive control can provide cardiovascular benefit. We know from previous subanalysis of the ORIGIN trial, that patients on glargine had better durable glycemic control. Another substudy from Diabetes Care show that the IMT carotids were a bit better in the glargine group. The question that needs to be asked is: "Is hypoglycemia is so bad with the lantus group that it is negating the positive benefit of glycemic control."
- How can you draw conclusions about nocturnal hypoglycemia. The absolute numbers between the lantus and control is 100 vs 18. 18!!
- If you treat your stable diabetics with lantus and titrate them to 95, you will get a high report of nonsevere and severe hypoglycemia, which obliterates any metabolic benefit you achieve using this regimen. You will also expect weight gain of 1.6kg of weight gain and more reports of new angina pectoris.