Should diabetes patients be on these new incretin drugs long term?
Gastroenterology. 2011 Jul;141(1):150-6. Epub 2011 Feb 18.
Abstract
BACKGROUND & AIMS: Glucagon-like peptide-1-based therapy is gaining widespread use for type 2 diabetes, although there are concerns about risks for pancreatitis and pancreatic and thyroid cancers. There are also concerns that dipeptidyl peptidase-4 inhibitors could cause cancer, given their effects on immune function.
METHODS:
- We examined the US Food and Drug Administration's database of reported adverse events for those associated with the dipeptidyl peptidase-4 inhibitor sitagliptin and the glucagon-like peptide-1 mimetic exenatide, from 2004-2009; data on adverse events associated with 4 other medications were compared as controls.
- The primary outcomes measures were rates of reported pancreatitis, pancreatic and thyroid cancer, and all cancers associated with sitagliptin or exenatide, compared with other therapies.
RESULTS:
- Use of sitagliptin or exenatide increased the odds ratio for reported pancreatitis 6-fold as compared with other therapies (P<2×10(-16)).
- Pancreatic cancer was more commonly reported among patients who took sitagliptin or exenatide as compared with other therapies (P<.008, P<9×10(-5)).
- All other cancers occurred similarly among patients who took sitagliptin compared with other therapies (P=.20).
CONCLUSIONS: These data are consistent with case reports and animal studies indicating an increased risk for pancreatitis with glucagon-like peptide-1-based therapy. The findings also raise caution about the potential long-term actions of these drugs to promote pancreatic cancer.



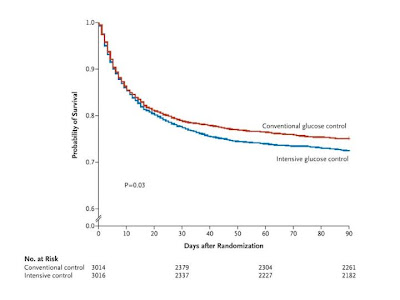
 40 mg per deciliter [2.2 mmol per liter]) was reported in 206 of 3016 patients (6.8%) in the intensive-control group and 15 of 3014 (0.5%) in the conventional-control group (P<0.001). There was no significant difference between the two treatment groups in the median number of days in the ICU (P=0.84) or hospital (P=0.86) or the median number of days of mechanical ventilation (P=0.56) or renal-replacement therapy (P=0.39).
40 mg per deciliter [2.2 mmol per liter]) was reported in 206 of 3016 patients (6.8%) in the intensive-control group and 15 of 3014 (0.5%) in the conventional-control group (P<0.001). There was no significant difference between the two treatment groups in the median number of days in the ICU (P=0.84) or hospital (P=0.86) or the median number of days of mechanical ventilation (P=0.56) or renal-replacement therapy (P=0.39). 