Noninvasive Quantification of Pancreatic Fat in Humans
The Journal of Clinical Endocrinology & Metabolism Vol. 94, No. 10 4070-4076Ildiko Lingvay, Victoria Esser, Jaime L. Legendre, Angela L. Price, Kristen M. Wertz, Beverley Adams-Huet, Song Zhang, Roger H. Unger and Lidia S. Szczepaniak
Objective: To validate magnetic resonance spectroscopy (MRS)
as a tool for non-invasive quantification of pancreatic triglyceride
(TG) content and to measure the pancreatic TG content in a diverse
human population with a wide range of body mass index (BMI)
and glucose control.
Methods: To validate the MRS method, we measured TG content in the pancreatic tissue of 12 lean and 12 fatty ZDF rats (ages 5–14 weeks) both by MRS and the gold standard biochemical assay. We used MRS to measure pancreatic TG content in vivo in 79 human volunteers. Additionally, to assess the reproducibility of the method, in 33 volunteers we obtained duplicate MRS measurements 1–2 weeks apart.
Results: MRS quantifies pancreatic TG content with high reproducibility and concordance to the biochemical measurement (Spearman’s rank correlation coefficient = 0.91). In humans, median pancreatic TG content was as follows: (1) normal weight and normoglycemic group 0.46 f/w%, (2) overweight or obese but normoglycemic group 3.16 f/w%, (3) impaired fasting glucose or impaired glucose tolerance group (BMI matched with group 2) 5.64 f/w%, and (4) untreated type 2 diabetes group (BMI matched with group 2) 5.54 f/w% (Jonckheere-Terpstra trend test across groups p <>
Conclusions: Human pancreatic steatosis, as measured by MRS,
increases with BMI and with impaired glycemia. MRS is a quantitative
and reproducible non-invasive clinical research tool which will
enable systematic studies of the relationship between ectopic
fat accumulation in the pancreas and development of type 2 diabetes.

--------------------
Comments:
How is pancreatic fat related to diabetes/IFG and how is it related to fat deposition in other organs? This intriguing study examines both animal models and humans to help understand the pathophysiology of fat deposition and its relationship to diabetes.
The figure above in A. shows glucose readings along with total pancreatic Triglycerides(pTG) as assessed by magnetic resonance spectroscopy. The pancreatic triglyceride measurements were correlated with a biochemical determination of TG for the rats - Fatty and Lean Zucker rats. There is a progressive increase in pTG along with glucose levels in the Fatty Zucker rats, but no change in either levels in the lean rats. Figure B shows the correlation between the MRS technique and biochemical determination.
Humans were divided into 4 groups and were scanned by MRS as well. The results are as in the abstract. Intereting findings included that blood glucose levels 2 hours after a glucose load were most closely correlated with pTG content. HBA1c, serum Tg, # of DM risk factors, age, BMI, fasting glucose, excercise, and dietary fat intake had either no or a weak relationship to pTG content.
Also of interest was the fact that there was little relationship between pancreatic and hepatic TG content (a previous report had shown a low correlation between hepatic and myocardial TG content).
What is not known is to what degree the TG deposition is related to Beta-cell damage/loss if at all? Also can medications, diet, weight loss or more severe caloric restriction reverse the pancreatic TG deposition and if so can this lead to a new B-cell production?


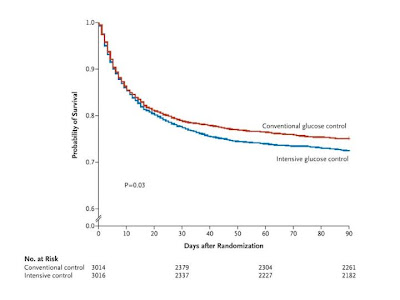
 40 mg per deciliter [2.2 mmol per liter]) was reported in 206 of 3016 patients (6.8%) in the intensive-control group and 15 of 3014 (0.5%) in the conventional-control group (P<0.001). There was no significant difference between the two treatment groups in the median number of days in the ICU (P=0.84) or hospital (P=0.86) or the median number of days of mechanical ventilation (P=0.56) or renal-replacement therapy (P=0.39).
40 mg per deciliter [2.2 mmol per liter]) was reported in 206 of 3016 patients (6.8%) in the intensive-control group and 15 of 3014 (0.5%) in the conventional-control group (P<0.001). There was no significant difference between the two treatment groups in the median number of days in the ICU (P=0.84) or hospital (P=0.86) or the median number of days of mechanical ventilation (P=0.56) or renal-replacement therapy (P=0.39). 

 krha, MD, DSC3
krha, MD, DSC3