Intensive versus Conventional Glucose Control in Critically Ill Patients
| |||||||||||||||||||||||||||||||||||||||||||||
Background
The optimal target range for blood glucose in critically ill patients remains unclear.
Methods
- Within 24 hours after admission to an intensive care unit (ICU), adults who were expected to require treatment in the ICU on 3 or more consecutive days were randomly assigned to undergo either intensive glucose control, with a target blood glucose range of 81 to 108 mg per deciliter (4.5 to 6.0 mmol per liter), or conventional glucose control, with a target of 180 mg or less per deciliter (10.0 mmol or less per liter).
- We defined the primary end point as death from any cause within 90 days after randomization.
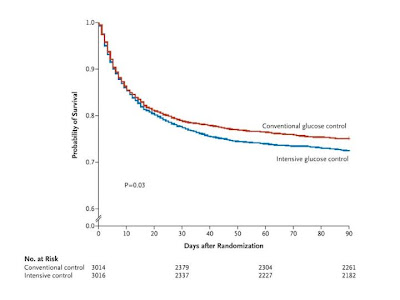
- Of the 6104 patients who underwent randomization,
- 3054 were assigned to undergo intensive control and
- 3050 to undergo conventional control; data with regard to the
- primary outcome at day 90 were available for 3010 and 3012 patients, respectively. The two groups had similar characteristics at baseline. A total of 829 patients (27.5%) in the intensive-control group and 751 (24.9%) in the conventional-control group died (odds ratio for intensive control, 1.14; 95% confidence interval, 1.02 to 1.28; P=0.02). The treatment effect did not differ significantly between operative (surgical) patients and nonoperative (medical) patients (odds ratio for death in the intensive-control group, 1.31 and 1.07, respectively; P=0.10). Severe hypoglycemia (blood glucose level,
 40 mg per deciliter [2.2 mmol per liter]) was reported in 206 of 3016 patients (6.8%) in the intensive-control group and 15 of 3014 (0.5%) in the conventional-control group (P<0.001). There was no significant difference between the two treatment groups in the median number of days in the ICU (P=0.84) or hospital (P=0.86) or the median number of days of mechanical ventilation (P=0.56) or renal-replacement therapy (P=0.39).
40 mg per deciliter [2.2 mmol per liter]) was reported in 206 of 3016 patients (6.8%) in the intensive-control group and 15 of 3014 (0.5%) in the conventional-control group (P<0.001). There was no significant difference between the two treatment groups in the median number of days in the ICU (P=0.84) or hospital (P=0.86) or the median number of days of mechanical ventilation (P=0.56) or renal-replacement therapy (P=0.39).
In this large, international, randomized trial, we found that intensive glucose control increased mortality among adults in the ICU: a blood glucose target of 180 mg or less per deciliter resulted in lower mortality than did a target of 81 to 108 mg per deciliter. (ClinicalTrials.gov number, NCT00220987 [ClinicalTrials.gov] .)
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.